Coronavirus 2021
COVID-19 is nearing the one-year mark in the United States with over 26.2 million cases to date. While cases in the United States are still doubling their daily totals, many people worldwide are experiencing pandemic fatigue. Just before the end of 2020, companies like Moderna and Pfizer, with help from many C & A Scientific customers, were able to provide a solution by developing a series of vaccines to eligible patients, depending on which state you are in. As of February 1st, 31.8 million vaccines have been administered in the United States and 1.7% of people have received their second dose.
This is the first massive step modern medicine has taken in tackling the virus. Paired with social distancing, wearing masks and heightened hygiene practices, medical professionals hope this moves us back into the direction of what we knew as everyday life.
C & A Scientific supports improving the health and minds of people worldwide and will continue developing products to help medical professionals create lifesaving solutions.
What Vaccines Are Available?
Currently, there are two vaccines available to the public in the United States, the Moderna vaccine and the Pfizer vaccine.
The Moderna vaccine contains ten doses per vial, each dose being 0.5 mL. The recommended dosage is two-doses separated by 28-days. This vaccine can be administered to patients over the age of 18.
The Pfizer vaccination contains six doses per vial, each dose being 0.3 mL. The recommended dosage is two doses taken 21-days apart. The Center for Disease Control (CDC) states that a Pfizer vaccine receiver must be 16 years of age or older to receive this vaccine.
Infants and children between the ages of 0-15 do not have access to the COVID-19 vaccine yet. There are currently ongoing studies to create a vaccine for kids. Researchers must make sure that the vaccine is safe enough for children before administering it. There are hopes of having a vaccine available for ages 0-15 by late 2021.
For more information on the available COVID-19 vaccines, please visit the CDC’s website here.
What is the Efficacy of the COVID-19 Vaccines?
Efficacy is the intended effect of something tested in a study, also stated as the effectiveness in a clinical trial, like the COVID-19 study. Researchers cannot determine the vaccine’s effectiveness until a significant amount of the population has been vaccinated.
The Moderna vaccine has a 94.1% efficacy, while the Pfizer vaccine has 95% effectiveness in the clinical trial. To achieve the effectiveness that was reached in the clinical trial, participants must receive both vaccines.
As more people get vaccinated, data on applied effectiveness will become available. Pfizer and Moderna are not the only companies producing a vaccine. The United States has signed deals with AstraZeneca, as well as Johnson & Johnson. These additional companies will produce the needed number of vaccines to vaccinate yourself and your families.
What is Herd Immunity and How Do We Achieve It?
Herd immunity is a form of indirect protection from an infectious disease. To achieve this, we must have a large amount of the population vaccinated. If enough people are vaccinated and immune to a virus, fewer people will get the infection.
According to the Lerner Research Institute, approximately 50 to 80% of the population will need to be vaccinated to reach the herd immunity threshold. In contrast, the flu needs between 33 to 44% vaccinated to get the herd immunity threshold.
The Bloomberg Vaccine Tracker allows you to track the percentage of people vaccinated, as well as the vaccine supply and other vaccine statistics for your state.
Common Concerns
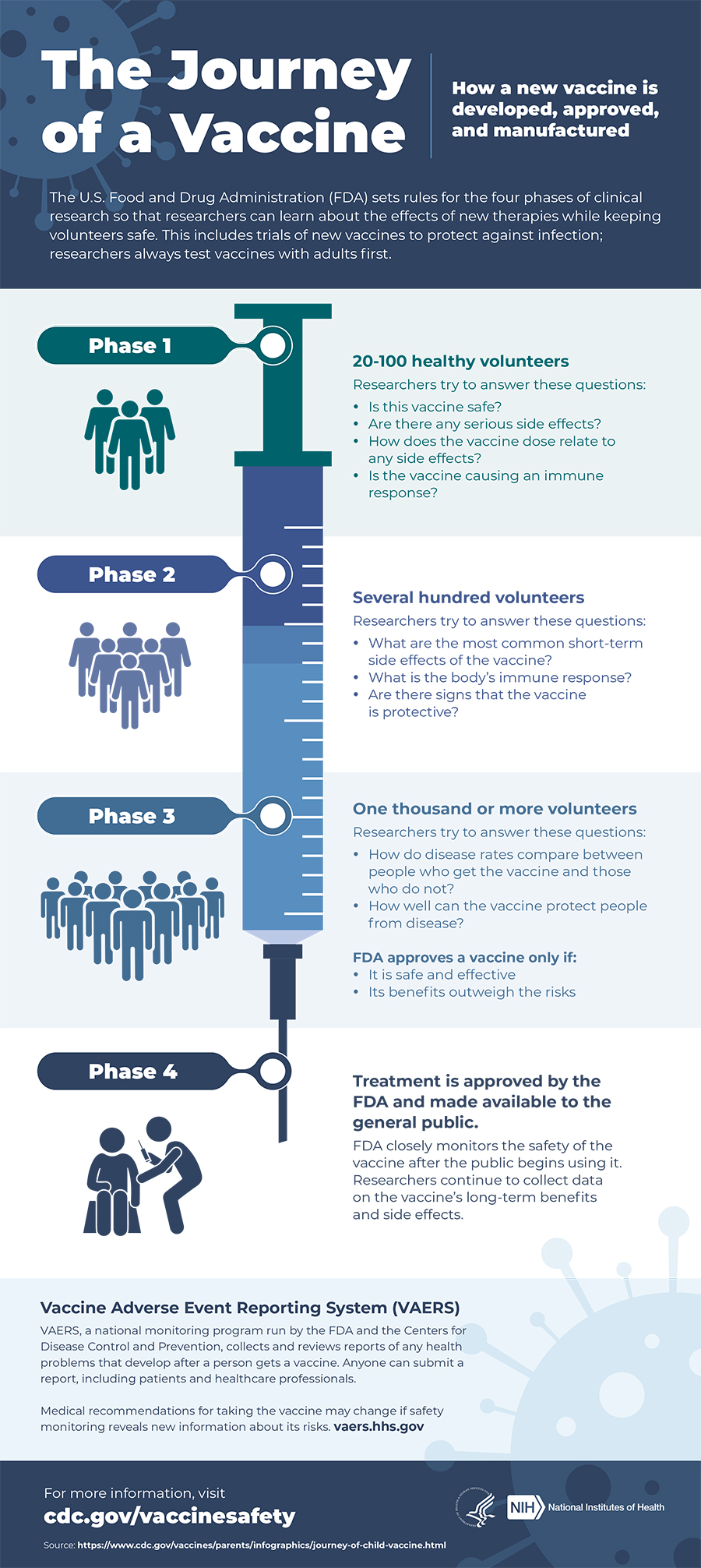
All vaccines go through clinical trials before being released to the public. This infographic shows the processes taken during vaccine creation to ensure a vaccine is safe for you and your family. Many people have expressed concerns about the adverse reactions that may be associated with the COVID-19 vaccine. According to the CDC, the COVID-19 vaccination is safe for individuals with common allergies but advise them to speak with their doctor before having a vaccination administered.
Unlike other vaccines, both the Moderna and Pfizer vaccinations do not contain traces of the virus or other infectious materials. Both vaccines are mRNA vaccines, which use proteins to teach tour body how to fight the virus. Some of the individuals vaccinated have reported soreness around the injection site and experienced a low-grade fever.
Where to Get Tested
The current supply of vaccinations is limited but will increase in the months to come. Visit the CDC website to see if you are qualified for the first round of doses or find your nearest vaccine location.
Doses purchased with U.S. taxpayer dollars will be administered at no cost. However, your health care provider may charge administrative fees.
C & A Scientific is a dedicated leader in improving the health and minds of people worldwide. We supply over 700 award-winning medical and STEM-inspired products to distributors and retailers looking for sensational customer service. Learn more about us and our story here.